Contact us :
UCSD-CNRS joint chemistry lab - UMI 3555
Address : University of California, San Diego, 5213 Pacific Hall, Department of Chemistry, 9500 Gilman Dr., La Jolla, CA 92093-0358
e-mail : guybertrand@ucsd.edu
Phone number : (858) 534 5412










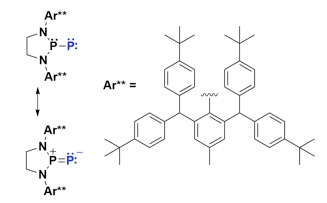
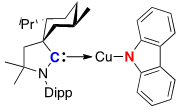
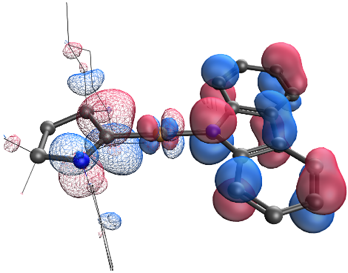
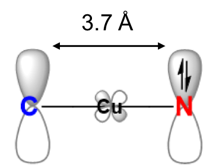
Eliminating nonradiativedecay in Cu(I) emitters: > 99%quantum efficiency and microsecond lifetime
OLED 2.0: Organic Light Emitting Diodes (OLED) are traditionally made using luminescent complexes of heavy metals such as iridium, platinum, and ruthenium. Achieving comparable performance from copper, an earth-abundant metal, requires overcoming weak spin-orbit coupling, as well as limiting high reorganization energies. In a recent report in Science, however, Jesse Peltier (left) from the Bertrand group at UCSD and Rasha Hamze (right) from the Thompson group at USC, thoroughly explored two-coordinate copper complexes that sandwich the metal between an amide ligand and a cyclic (alkyl)(amino)carbene ligand (CAAC) and measured a nearly perfect luminescence efficiency. They used this property to produce a prototype blue organic light-emitting diode.
Science 2019, 363, 601.
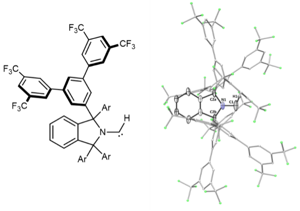
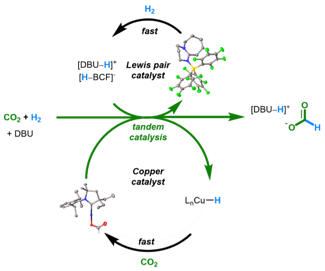
A Crystalline Doubly Oxidized Carbene
The chemistry of carbon is governed by the octet rule, which refers to its tendency to have eight electrons in its valence shell. However, a few exceptions do exist. The trityl radical (Ph3C∙) and carbocation (Ph3C+) possess seven and six valence electrons, respectively. Carbenes (R2C:), two-coordinate octet-defying species, formally possess six valence electrons. Can we undress the carbene further by removing its non-bonding electrons? As published in Nature and highlighted in C&EN, we have synthesized a crystalline doubly oxidized carbene (R2C2+) formally possessing just four valence electrons.
Nature 2023, 1.
DOI: 10.1038/s41586-023-06539-x
C&EN 2023,

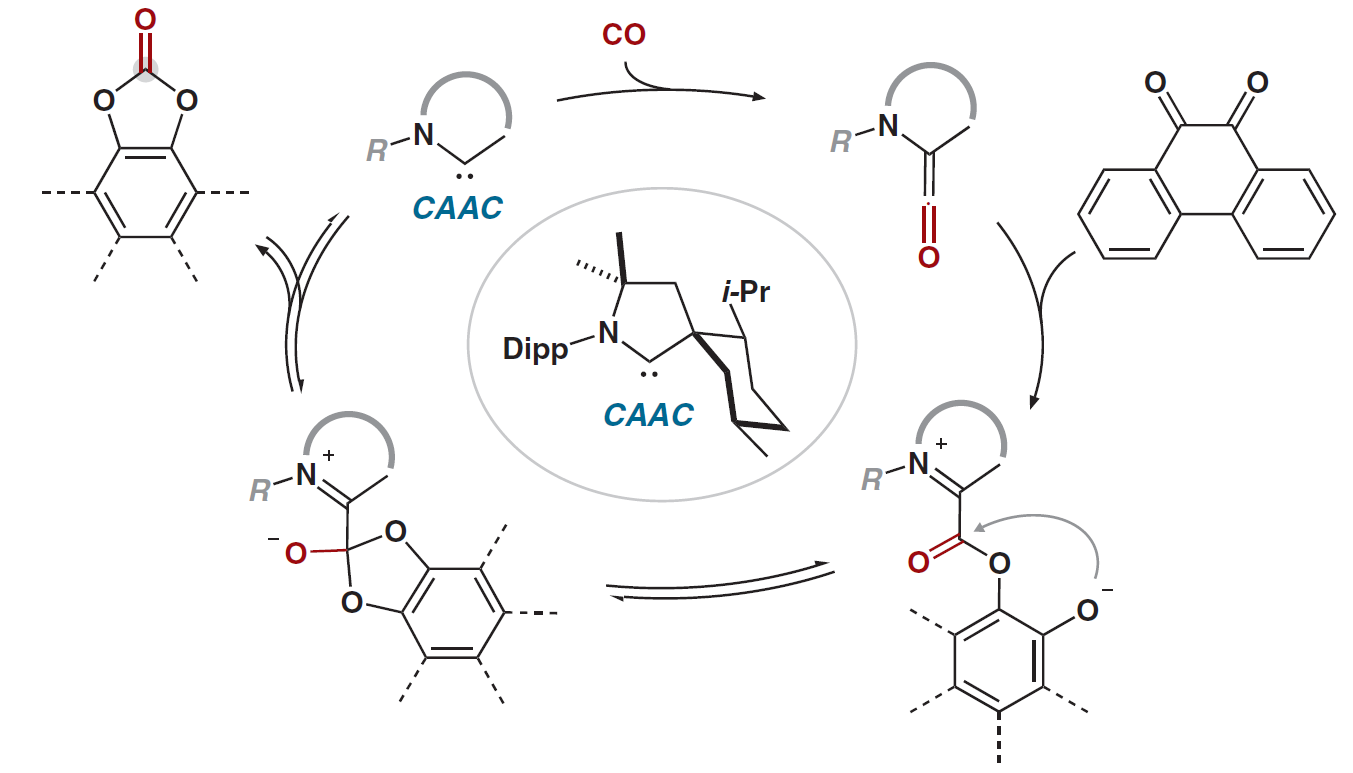
Ambiphilic Carbene Mimicks Transition-Metal Catalysts in the Activation of Carbon Monoxide
The activation and catalytic transformation of carbon monoxide by transition metals is well known. However, within the realm of organocatalysis the former behavior is rare while the latter is entirely unknown - until now. As published in JACS and highlighted in Synfacts, we have succesfully demonstrated a metal-free, carbene-catalyzed carbonylation with CO. Insights from this discovery will pave the way for further developments in metal-free catalysis.
Synfacts 2021, 17, 0088.
JACS 2020, 142, 18336–18340.


Contact us :
UCSD-CNRS joint chemistry lab - UMI 3555
Address : University of California, San Diego, 5213 Pacific Hall, Department of Chemistry, 9500 Gilman Dr., La Jolla, CA 92093-0358
e-mail : guybertrand@ucsd.edu
Phone number : (858) 534 5412
Financial support :